Veeva Vault CTMS
Enable Faster Trials
Equip study teams with role-based dashboards and intuitive navigation to improve productivity and speed trial execution.
Improve Decision-Making
Enable proactive closed-loop issue management and improve strategic trial planning with a real-time view of trial status.
Streamline Clinical Operations
Provide one system of record for shared study start-up, TMF, CTMS, and payments content, improving efficiency and unifying operations.
Features
Study Planning and Management
Plan and track study milestones across investigator-initiated studies and trial activities to optimize resources and proactively plan for events such as aligning clinical supply arrival with the site initiation visit or assessing site performance across studies. Vault CTMS enables seamless subject visit planning based on categories such as protocol, visit frequency, and procedures.
Subject Recruitment Planning
Plan the number of subjects that will be screened, enrolled, or randomized within a study and get a comprehensive view at the study, study country, and site levels. With metrics that update with actual recruitment data, you can track subject enrollment against goals to ensure studies are on time.
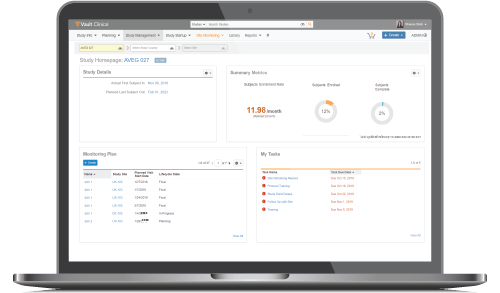
Site Monitoring
Manage all aspects of routine monitoring visits – pre-study, site initiation, interim monitoring, and closeout – in Vault CTMS. CRAs can view key information such as enrollment metrics and violations at-a-glance on the CRA homepage, quickly author new monitoring visit reports, and track onsite monitoring activities, all in one application.
Investigator Relationship Management
Empower study teams with an accurate and complete view of interactions between sponsors, CROs, investigators, and site personnel. Track site communication logs, site monitoring visits, resources assigned to sites, and more to strengthen collaboration and improve study execution.
Risk Management
Focus on the critical risks within your study and allocate resources more effectively with risk-based study management. Generate a study-level Risk Assessment for any of your studies from a risk library you create in Vault CTMS. Create risk templates that can be reused across studies and calculate risk scores based on impact, probability, and detectability.
Study Oversight
Monitor study performance, track CRO activity, and maintain communication logs to help ensure regulatory compliance with ICH/GCP guidelines for study oversight. CRAs can also capture and track protocol deviations for effective issue management.
Site Payments
Speed payments to clinical research sites and provide financial visibility to all study partners with Vault Payments. Designed to support complex trials, Vault Payments enables sponsors and CROs to pay sites faster and more accurately.
Interactive Reports and Dashboards
Drill down through interactive dashboards to narrow in on trial status and easily identify sources of delays. Take corrective action directly from reports to restore momentum.
Connection with Vault EDC
Leverage select operational data from Vault EDC for reporting within Vault CTMS. CTMS users can report on the status of subjects and dates captured in Vault EDC, as well as navigate to a subject’s casebook.
Unified Clinical Operations
Streamline end-to-end clinical trial processes, from site activation to study closeout, in one unified system. Trial information, such as trip reports and SQVs, are entered once and immediately available across Vault Study Startup, Vault eTMF, and Vault CTMS. With a single source of truth across the full trial lifecycle, teams increase visibility, quality, and speed of execution.
Connected Sponsor and Site Operations
Automate information sharing across trial partners, processes, and systems for better collaboration and faster trials with Veeva Site Connect.